Manganese is a transitional metal but which unlike most metals of this family it does not form many coloured ppt or complex solutions It forms a white ppt with the Ammonium Hydroxide and Sodium Hydroxide insoluble in xs which turns to a brown ppt on contact with air. An ion with a net negatively charge.

Collection Of Writing Chemical Formulas For Compounds Worksheet Writing Worksheets Chemistry Worksheets Covalent Bonding Worksheet
And repel cations like calcium magnesium etc.
. Anion exchange capacity increases with the decrease in soil pH. A cation is a positively charged ion and most inorganic chemical contaminants are cations such as. They are formed when a metal loses electrons and nonmetals gain those electrons.
Hydrogen H and H- Carbon which can form many anions and cations Oxygen O-2. Anions 1-acetate C 2 H 3 O 2. Terms in this set 33 What is an Anion.
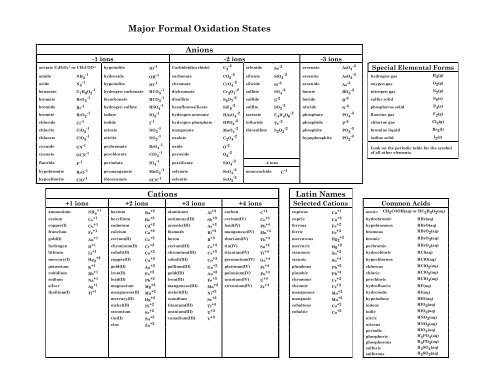
Anions on the other hand gain new ones to become larger in size and have a positive charge. Fe2 Colbalt II Co2 Cadmium. Common Cations and Anions Name Formula Charge Name Formula Charge Name Formula Charge aluminum Al 3 3 magnesium Mg 2 2 carbonate CO 3 2 2 ammonium NH 4 1 manganese II Mn 2 2 chlorate ClO 3 1 barium Ba 2 2 manganese III Mn 3 3 chloride Cl 1 cadmium Cd 2 2 mercury I mercurous See note Hg 2 2 2 X 1 chromate CrO 4.
Is it Anion or Cation and what is the formula. Is mn a cation or anion. It is a divalent metal cation a manganese cation and a monoatomic dication71FDA Pharmacological Classification.
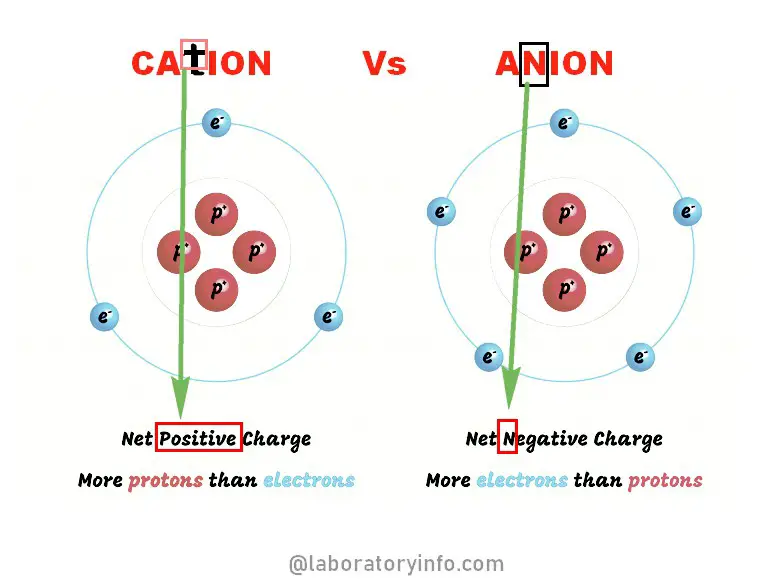
The electrostatic attraction between positives and negatives attracts the particles to each other and creates an ionic compound for example sodium chloride. In order to be considered a cation or anion there must be charge involved. Anion exchange capacity of the soil is usually very low ie a few tenth of a mill equivalent per 100gms of a soil.
Common Cations and Anions Name Formula Charge Name Formula Charge Name Formula Charge aluminum Al 3 3 magnesium Mg 2 2 carbonate CO 3 2 2 ammonium NH 4 1 manganese II Mn 2 2. As this ionic substance reacts with the electrodes it generates electrical current. In between the electrodes is an electrolyte liquid or gel that contains charged particles ions.
Therefore cations and anions attract each other and form an ionic bond between them. By itself oxygen is neutral does not have a charge. In order to be considered a cation or anion there must be charge involved.
Boron BO3-3 Anion Chlorine Cl- Anion Manganese Mn2 Cation Iron Fe2Fe3 Cation Nickel Ni2 Cation Copper CuCu2 Cation Zinc Zn2 Cation Molybdenum MoO4-2 Cation Non-fertilizer elements obtained through the air and water. Cations are smaller than the parent atom because theyve lost electrons. Oxide anion sulfide anion.
The gain of one or more electrons. In Newtons law of attraction opposite attracts. What are the names of the cations and anions.
2 4 7 26. IronIII a 3 charge. What are the two types of ions.
They have a negative electrical charge where the neutral atom has gained one or more electrons. Ca2 Iron II Cation. In single use dry cell batteries zinc is commonly used as the anode whilst manganese dioxide is a popular choice for the electrolyte cathode.
They treated the solid manganese dioxide medium as a homogeneous medium composed of cations of MnIV and MnIII and oxide and hydroxyl anions Figure 2. Electrolytic manganese dioxide has little semiconductor-like properties implying that the electron in the matrix is delocalized to some extent and if there is a trivalent manganese cation it has a possibility to. ManganeseIII Mn3 4 tinIV Sn4 nickelIV Ni4 leadIV Pb4 Roman numeral notation indicates charge of ion when element commonly forms more than one ion.
On the other hand a metal reacts with a nonmetal to form an ionic bond. This is already a quite good indication of the presence of Manganese. They have a net electrical charge that can be positive where the neutral atom has lost one or more electrons.
Ammonium NH4 calcium Ca2 copper Cu2 magnesium Mg2 manganese Mn2 potassium K and zinc Zn2. It has a role as a cofactor. Mn2 Copper II Cation.
It is a phosphate ion and a trivalent inorganic anion. Anion anode cathode cation electron lattice energy negatively - charged ion nuclear charge polarity positively charged ion proton valence orbitals. How are a cation and anion formed.
It is a conjugate base of a hydrogenphosphate. Negatively charged ions are referred to as anion while positively charged ions are cations. Soils with net positively charged colloids adsorb anions like phosphate sulphate etc.
Which are then lost by leaching. For example ironII has a 2 charge. Manganese 2 is a divalent metal cation in which the metal is manganese.
One of them concerns the of manganese ions in our CS-NPs is larger than 2 the value oxidation of Mn ions which could easily occur when the NPs expected for an ideal Mn ferrite structure2021 A more detailed are coprecipitated in a highly basic medium25 Then as has analysis leads to the energy of the central peak located at been observed. Cesium cation mercury cation.

Aleks Deducing The Ions In A Polyatomic Ionic Compound From Its Empirical Formula Youtube
0 Comments